Photo credit: Flickr user Caramosca (CC BY-NC 2.0) ; https://flic.kr/p/2bLPu4q
Passing
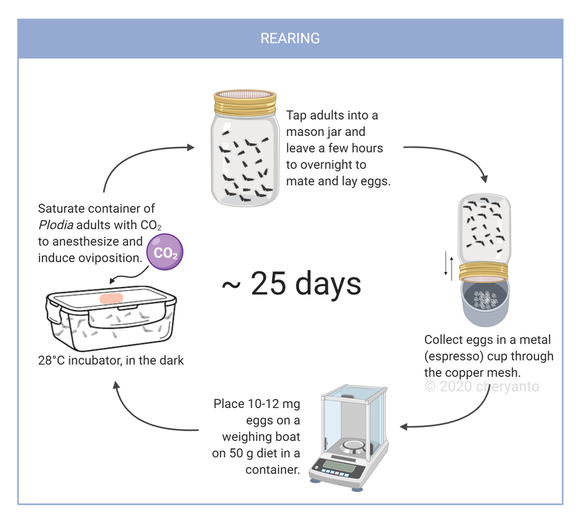
- Prepare 45-50 g of Plodia diet in a modified container (copper mesh soldered to the lid for ventilation). Pack it tightly down to the bottom of the container with a gloved fist.
- Measure 10-12 mg of Plodia eggs on a small weighing boat by tapping the metal cup gently.
Plodia eggs disperse easily by sudden air change in airflow (through breathing or moving them too fast) and electrostatic effects (dry plastic, nitrile gloves). They are still used during handling but make sure to kill any residue especially on the gloves, the bench, and the weighing scale if the eggs do disperse. - Place the weighing boat on top of the packed food.
- Cover the copper mesh of the stock container with filter paper and tape around it for label.
- Information on the label: Pi_(strain) [date passed] [10-12 mg eggs]/[45-50 g diet]
- Leave at 28'C until they hatch and become larvae.
- Check every 3-4 days for any signs of infection (yeast), food depletion (overly wandering larvae), or overproduction of silk.
Optional: Pupal isolation can be done around 15 days after egg laying. Place some cut cardboard for the larvae to pupate in ("hotels"). Separate them as individual manually (e.g. for sexing) or as hotels into another container (e.g. to rescue from mold infection). - At around 25-28 days, adults should have emerged. Leave freshly emerged adults 1-2 days to mate then start the synchronized oviposition procedure.
Synchronized OvipositionMake sure the inside of the mason jar is completely dry. Eggs may adhere to damp spots inside the jar.
|
Cleaning and Disposal
Freezing at -20'C is efficient to kill Plodia at adult, larval, and egg stage. Alternatively, eggs can be killed by submerging in 30% bleach overnight. To clean containers, simply rinse in a larger bleach container then use soap and brush to scrape any silk, food, and eggs residue away. Dry completely prior to next usage. Contact the biological safety officers in your institution and ensure compliance of your protocol with the local regulation for disposal.
Infection
The most common stock infection problem comes from mold, which can stem from either the food being too dry, leading larvae to expel wet droppings. This will in turn raise the humidity inside the container and harbor mold. It is recommended to split a fresh dry food batch into a few food containers since repeated opening and closing will dry out food rapidly. Apart from that, during larval stage, stressed larvae may loom silk from around the cover mesh and lid. Thick silk may even cover the entire culture (thin silk is not a problem). Too much silk production in the container can result in overheating and moisture build up that leads to mold infection.